Zelsuvmi: A New Topical Treatment for Molluscum Contagiosum Approved on January 5, 2024
What is it prescribed for?
ZELSUVMI is indicated for the topical treatment of molluscum contagiosum (MC) in adults and pediatric patients 1 year of age and older
What is the name of the drug and what does it do?
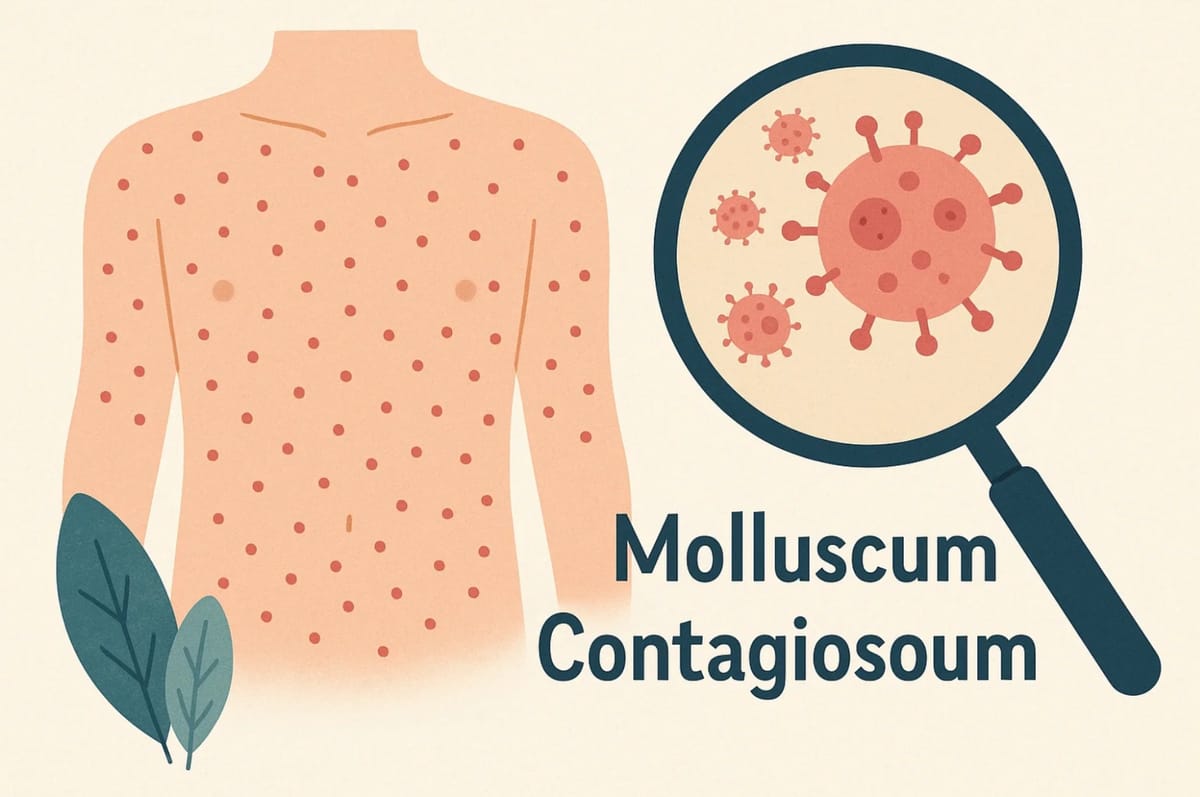
Zelsuvmi (pronounced zel-SOOV-mee), (generic name: berdazimer), is a medication indicated for the topical treatment of molluscum contagiosum (MC) in adults and pediatric patients 1 year of age and older. Molluscum contagiosum is a viral skin infection that causes raised, pearl-like papules or nodules on the skin.
How does it work?
Zelsuvmi is a nitric oxide (NO) releasing agent. Nitric oxide has antimicrobial properties and plays a role in the body's defense against infections. When applied to the skin, Zelsuvmi releases nitric oxide, which helps to clear the molluscum contagiosum lesions by exerting its antimicrobial effects and possibly enhancing the local immune response.
What did the research discover?
Clinical trials demonstrated that Zelsuvmi is effective in treating molluscum contagiosum. In three randomized, double-blind, vehicle-controlled trials involving 1,596 subjects, Zelsuvmi showed a higher rate of complete clearance of MC lesions compared to the vehicle. In Trials 1 and 2, 32.4% and 30.0% of subjects treated with Zelsuvmi achieved complete clearance at Week 12, compared to 19.7% and 20.3% in the vehicle groups, respectively. This demonstrates the effectiveness of Zelsuvmi in reducing and eliminating MC lesions.
What are some of the side effects?
- Application site pain (burning or stinging sensations)
- Application site erythema (redness)
- Application site pruritus (itching)
- Application site exfoliation (peeling)
- Application site dermatitis
- Application site swelling
- Application site erosion
- Application site discoloration
- Application site vesicles (blisters)
- Application site irritation
- Application site infection
- Vomiting
- Upper respiratory tract infection
- Pyrexia (fever)
What are the dosage recommendations and how is it prescribed?
Zelsuvmi is supplied as a topical gel that must be mixed from two components before application. The recommended dosage and administration instructions are as follows:
- Dispense equal amounts (0.5 mL) of gel from Tube A (berdazimer gel) and Tube B (hydrogel) on the provided dosing guide.
- Mix the gels together on the dosing guide and immediately apply a thin, even layer to each MC lesion once daily.
- Allow the gel to dry for 10 minutes and avoid swimming, bathing, or washing the treated areas for 1 hour after application.
- Wash hands after applying Zelsuvmi, unless hands are being treated.
- Zelsuvmi is for topical use only and not for ophthalmic, oral, or intravaginal use.
Source:
EPIH SPV, LLC. Zelsuvmi (berdazimer) topical gel. Prescribing Information. 2024. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/217424s000lbl.pdf