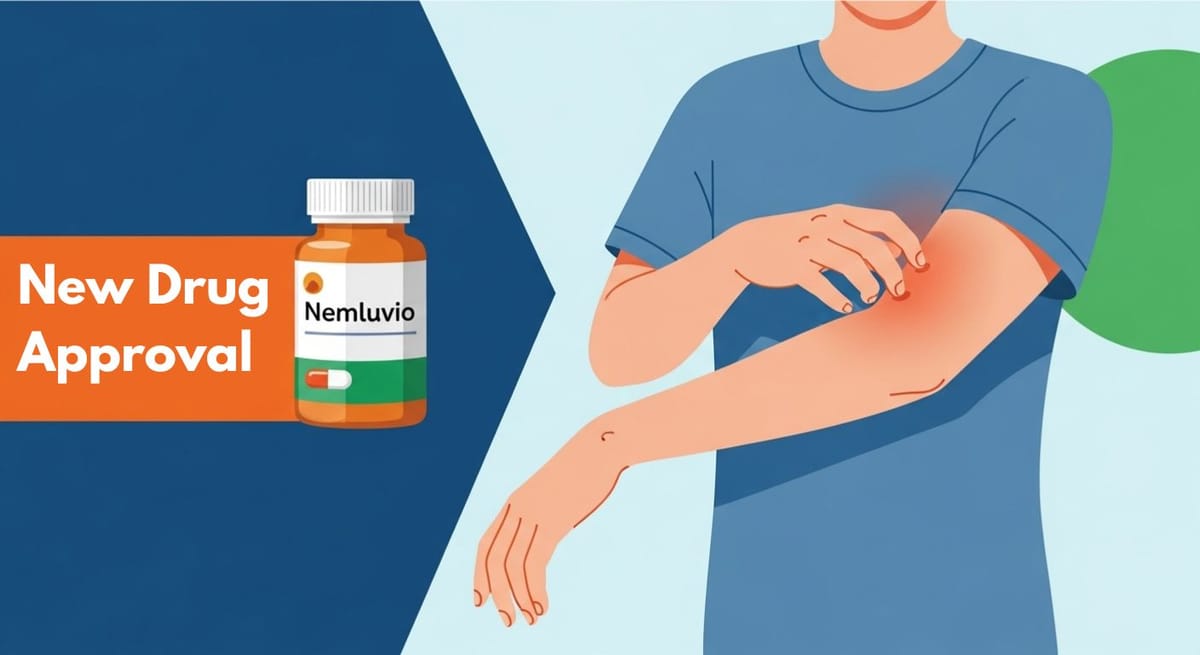
Nemluvio: a New Way to Treat Chronic Itchy Skin Condition
What is the name of the drug and what does it do?
Nemluvio (pronounced Nem-LOO-vee-oh), generic name nemolizumab-ilto, is a medication used to treat adults with prurigo nodularis. This is a chronic skin condition that causes extremely itchy, hard bumps to form on the skin. Nemluvio helps reduce itchiness and improves the appearance of the skin by targeting the underlying cause of inflammation.
How does it work?
Nemluvio is a monoclonal antibody that blocks the interleukin-31 receptor, which plays a key role in causing itchiness and inflammation in prurigo nodularis. By stopping this receptor from being activated, Nemluvio helps to calm the skin, reduce itching, and improve healing of the skin lesions.
What did the research discover?
In clinical trials, Nemluvio showed significant improvement in reducing itch and healing skin lesions for people with prurigo nodularis. In two major studies, a higher percentage of patients treated with Nemluvio experienced a reduction in itch severity and skin improvement compared to those who received a placebo. For example, in one study, 22% of patients treated with Nemluvio achieved clear or almost clear skin compared to just 2% of those on placebo.
What are some of the side effects?
The most common side effects of Nemluvio include:
- Headaches
- Atopic dermatitis (a type of eczema)
- Eczema or eczema nummular (circular patches of irritated skin)
Some people may also experience allergic reactions, which can include swelling of the face or lips, breathing problems, or dizziness. It’s important to seek medical attention if any serious side effects occur.
What are the dosage recommendations and how is it prescribed?
Nemluvio is given as an injection under the skin (subcutaneous). The dose depends on a person’s weight:
- For patients weighing less than 90 kg (198 pounds): The initial dose is 60 mg (two injections of 30 mg), followed by 30 mg every 4 weeks.
- For patients weighing 90 kg or more: The initial dose is 60 mg (two injections of 30 mg), followed by 60 mg every 4 weeks.
Patients or caregivers can learn to administer Nemluvio themselves after proper training from a healthcare provider.
Source
U.S. Food and Drug Administration. Full prescribing information: [Label for BLA 761390]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761390s000lbl.pdf. Accessed 2024 Nov 20.