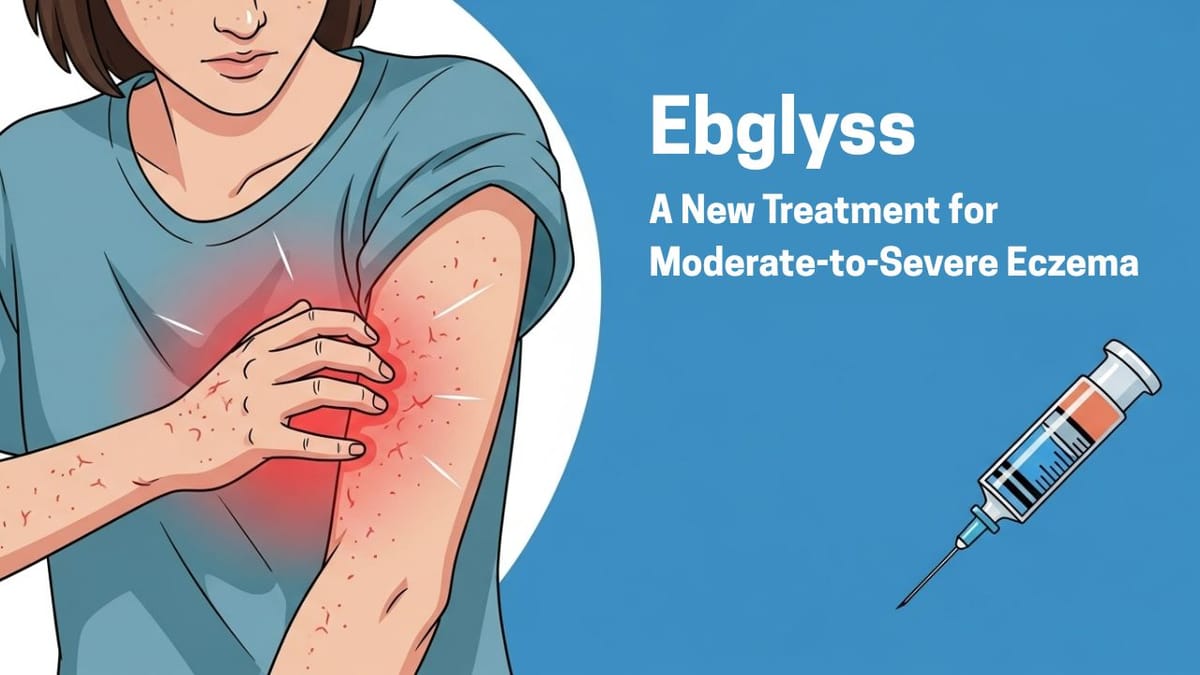
Ebglyss: A New Treatment for Moderate-to-Severe Eczema
What is the name of the drug and what does it do?
Ebglyss (pronounced EHB-glihs), generic name lebrikizumab-lbkz, is a medicine used to treat moderate-to-severe atopic dermatitis, also called eczema. It is approved for adults and children 12 years and older who weigh at least 88 pounds. Ebglyss is for people whose eczema cannot be controlled with creams or ointments or for those who cannot use those treatments.
How does it work?
Ebglyss works by targeting a specific protein in the body’s immune system called interleukin-13 (IL-13). IL-13 is one of the main causes of inflammation in eczema, leading to red, itchy, and irritated skin. By blocking this protein, Ebglyss reduces the inflammation, which helps the skin heal and makes it less itchy.
What did the research discover?
Clinical trials involving more than 1,000 people with moderate-to-severe eczema showed that Ebglyss was highly effective. After 16 weeks of treatment, 43% of people who used Ebglyss achieved clear or almost clear skin, compared to just 13% of those who didn’t receive the drug. Many patients also experienced a 75% reduction in eczema symptoms, with some seeing as much as a 90% improvement. Additionally, 46% of patients reported significant relief from itching, compared to only 13% in the placebo group. These results show that Ebglyss not only improves the appearance of the skin but also enhances the quality of life for those living with severe eczema.
What are some of the side effects?
The most common side effects of Ebglyss include:
- Eye problems: Redness, swelling, or itching in the eyes and eyelids.
- Injection site reactions: Mild redness or swelling where the shot is given.
- Shingles (herpes zoster): Reactivation of the chickenpox virus.
Rare but serious allergic reactions can happen. Symptoms to watch for include trouble breathing, swelling, hives, or a severe rash. If these occur, seek medical help immediately.
What are the dosage recommendations and how is it prescribed?
Ebglyss is given as an injection under the skin.
- Starting dose: Two 250 mg injections (500 mg total) at Week 0 and Week 2.
- Follow-up doses: After the initial doses, one 250 mg injection is given every 2 weeks until Week 16.
- Maintenance dose: Once symptoms improve, patients receive one injection every 4 weeks.
Patients or caregivers can be trained to give these injections at home, or they can visit a healthcare provider for the treatment.
Source:
Eli Lilly and Company. Full prescribing information: EBGLYSS (lebrikizumab-lbkz) injection. Available from: https://www.fda.gov/drugsatfda. Accessed 2024 Nov 21.