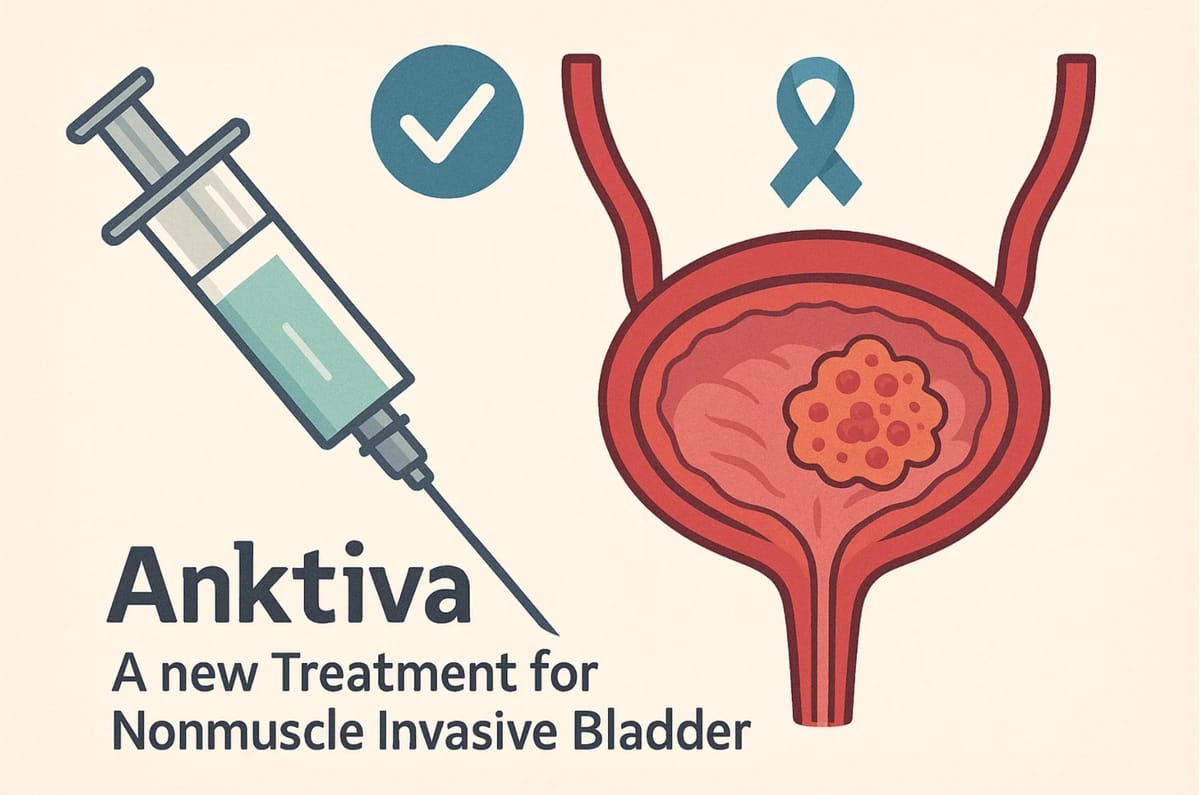
Anktiva: A New Treatment for Nonmuscle Invasive Bladder Cancer Approved on April 22, 2024
What is it prescribed for?
ANKTIVA is indicated with Bacillus Calmette-Guérin (BCG) for the treatment of adult patients with BCGunresponsive nonmuscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS) with or without papillary tumors.
What is the name of the drug and what does it do?
Anktiva (pronounced ank-TEE-vah), (generic name: nogapendekin alfa inbakicept), is a medication used in combination with Bacillus Calmette-Guérin (BCG) for the treatment of adult patients with BCG-unresponsive nonmuscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS), with or without papillary tumors.
How does it work?
Anktiva is an interleukin-15 (IL-15) receptor agonist. IL-15 is a cytokine that enhances the activation and proliferation of natural killer (NK) cells and T-cells, which are crucial for the immune response against tumors.
By activating these cells, Anktiva helps to boost the body's immune response to attack and kill cancer cells in the bladder.
What did the research discover?
Clinical trials have shown that Anktiva, when used with BCG, is effective in treating NMIBC. In the QUILT-3.032 trial, 62% of patients achieved a complete response, meaning there were no signs of cancer after treatment.
Many patients maintained their response for 12 months or longer, with some showing a response lasting up to 47 months. This demonstrates the drug's potential to provide long-term control of bladder cancer.
What are some of the side effects?
- Increased creatinine
- Dysuria (painful urination)
- Hematuria (blood in urine)
- Urinary frequency
- Micturition urgency (urgent need to urinate)
- Urinary tract infection
- Increased potassium levels
- Musculoskeletal pain
- Chills
- Pyrexia (fever)
What are the dosage recommendations and how is it prescribed?
Anktiva is administered intravesically, meaning it is instilled directly into the bladder. The recommended dosage regimen is as follows:
- Induction: 400 mcg administered intravesically with BCG once a week for 6 weeks. If a complete response is not achieved at month 3, a second induction course may be given.
- Maintenance: 400 mcg administered intravesically with BCG once a week for 3 weeks at months 4, 7, 10, 13, and 19. For patients with an ongoing complete response at month 25 and later, additional maintenance instillations with BCG may be given once a week for 3 weeks at months 25, 31, and 37.
Source:
Altor BioScience, LLC, an indirect wholly-owned subsidiary of ImmunityBio, Inc. Anktiva (nogapendekin alfa inbakicept) solution, for intravesical use. Prescribing Information. 2024. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761336s000lbl.pdf